Site Approval
Site shall be away from filthy surroundings and shall not be
adjacent to an open sewerage, drain, public lavatory or any
factory which produces a disagreeable or obnoxious odor or
fumes or large quantities of shoot.
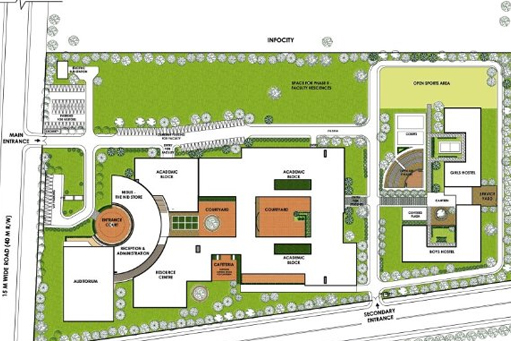
Layout Plan
Layout plans are the most important part of pharmaceutical industry .we made it on the best standards of WHO, ICH and FDA.
Construction
We have the best engineers in our team who made the structural drawings on the basis of your soil testing report and take care all the building guidelines and future prospects.
Equipment Import
PCP has the vast experience and contacts with the machine manufacturers .we import CGMP compliance machinery for the factories from CHINA and from EUROPE .which is entirely on the demand of the principal what it wants. We import machine, install it and then make test batches and then give after sale service too.
Installation of Equipment
We have the Vander qualification on which we make your installation qualify for the inspection after installing, testing .
Hiring of Staff
PCP is with you in selecting the best HR for Your factory for production and QC .our experience panel interviewers will help you in this regard.
Final Inspection
Dear PCP make you ready for the inspection by making SOPs for production and QC and JD for the inspection and making your staff fully trained on their jobs.
Manufacturing licensee
After the successful inspection of your unit PCP will finally get the manufacturing license which is your ultimate goal and PCP is there for you to give assistance and making this dream come true.
Registration of Products
PCP with the expertise in this field will provide the dossiers and will give you formulation of the products and assist you in choosing best molecules which are available internationally and that will help you boost your sales and profitability.